In vivo manipulation of human gut Bacteroides fitness by abiotic oligosaccharides – Nature Chemical Biology
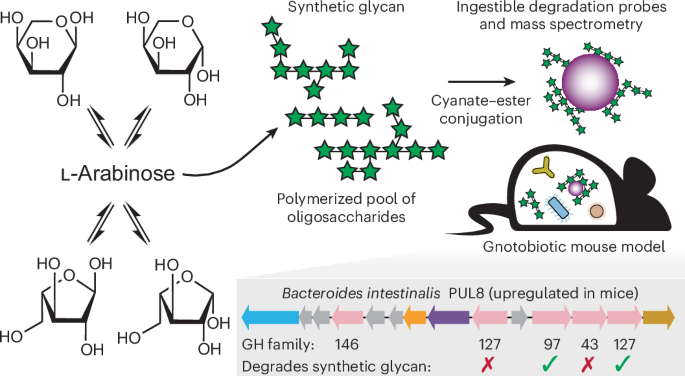
Preparation and characterization of synthetic oligosaccharides
Synthesis
SGs were synthesized according to procedures detailed in ref. 7. Monosaccharides (percent wt:wt ratio as described in Supplementary Table 1 to a final weight of 100 g) were combined with Dowex Marathon C (7.0 g, 5% dry weight ratio to monosaccharides, 29% moisture content) and 30 ml deionized water in a 1,000 ml three-necked round-bottom flask equipped with an overhead stirrer, thermocouple plug and short-path distillation head. The mixture was stirred continuously at 100 rpm using a glass stirring shaft equipped with a Teflon halfmoon paddle. The reaction mixture was run at 130 °C for 4 h. To quench the reaction, 60 ml deionized water was added to the mixture. The Dowex resin was removed by vacuum filtration through a fritted-glass filter. For SG1-7, the resulting solutions were diluted to 25 degree Brix (°Bx) and purified by ethanol precipitation. To do so, each solution was slowly poured into absolute ethanol to form a cloudy solution with a final water:ethanol ratio of 1:9 (vol:vol). The cloudy solution was then centrifuged (2,100g, 2 h, 5 °C). The supernatant was removed, and the precipitate was collected and dissolved in water. Residual ethanol was removed under reduced pressure. The solution was frozen at −20 °C and lyophilized to yield the final product as a white powder.
For SG10, a 25 °Bx solution (100 ml, deionized water) was poured into vigorously stirred United States Pharmacopeia-grade ethanol (900 ml) at a rate no greater than 10 ml min−1. After the addition was completed, the precipitated solids were allowed to stir for an additional 15 min at room temperature. The suspension was centrifuged (2,100g, 4 h, 5 °C), and the resulting pellet was isolated by decanting supernatant. The pellet was redissolved in deionized water to a final concentration of 25 °Bx and reconcentrated on a rotatory evaporator to >65 °Bx to remove ethanol. This process was repeated to ensure removal of residual ethanol. The resulting syrup was diluted to 20 °Bx, cooled to −78 °C and lyophilized to yield SG10 preparation 2 as a white powder. Next, SG10 preparation 2 (~4 g, ~50 °Bx) was loaded onto a Teledyne ISCO RediSep Rf Gold Amine column (55 g stationary phase) using a luer tip syringe. The sample was purified on a Biotage Isolera equipped with an evaporative light scattering detector using a 20/80 to 50/50 (vol/vol) deionized water/acetonitrile mobile phase gradient over 55 column volumes. After the monomer fraction completely eluted at ~16 column volumes, the mobile phase was set to 100% deionized water until the remainder of the oligosaccharide composition eluted and was collected. The monomer-free fractions were concentrated by rotary evaporation to ~20 °Bx, cooled to −78 °C and lyophilized to yield the SG10 as a white powder (preparation 1).
Determination of the physicochemical properties of the eight SG pools
Each SG was redissolved in deionized water to 30 mg ml−1. The solution was filtered (0.2 mm), and 10 μl was injected to an Agilent 1100 HPLC system equipped with a refractive index detector, a guard column (Agilent PL aquagel-OH (7.5 × 50 mm, 5 µm); PL1149-1530) and two SEC columns (Agilent PL aquagel-OH (7.5 × 300 mm, 5 µm); PL1120-6520) connected in tandem. The mobile phase was 0.1 M NaNO3, the run time was 28 min, the flow rate was 0.9 ml min−1 and the column and refractive index detector were kept at 40 °C. Sample peak areas were integrated, and the weight-average molecular mass, number average molecular mass, mean DP, PDI and purity (in terms of percentage of oligosaccharide with a DP of at least 2) were determined using Agilent Cirrus GPC/SEC software (v3.4.2). A calibration curve was generated from polymer standard solutions (10 mg ml−1) of d-(+)-glucose (peak molecular weight (Mp) 180), maltose (Mp 342), maltohexaose (Mp 990), nominal Mp 6100 pullulan standard, nominal Mp 9600 pullulan standard, nominal Mp 22000 pullulan standard and nominal Mp 43000 pullulan standard (Carbosynth).
Ex vivo fermentation studies of SGs with human fecal samples
Fecal biospecimens were collected with informed consent from donors7. Briefly, donors collected feces in a sample collection unit that was immediately sealed and placed on ice. Within 4 h, the sample collection units were transferred into an anaerobic chamber, unsealed, and the fecal biospecimen was transferred into filtered blender bags (Interscience) where they were diluted in phosphate-buffered saline and glycerol to a 20% slurry (wt:wt) containing 15% glycerol (wt:wt). Diluted samples were homogenized (Interscience, 032230), cooled to −78 °C and stored at −80 °C. Fecal communities were cultured under anaerobic conditions at 37 °C in Clostridial Minimal 3 (ref. 7) in 96-well plates (Corning, 3860) that had been sealed using a Breathe-Easy sealing membrane (Sigma-Aldrich, Z380059), with a sole carbon source supplemented at 5 mg ml−1. Growth was monitored by repeated measurements of optical density at 600 nm and culture pH51 using a Biotek Synergy H1 multimode plate reader outfitted with a Biostack 4 plate stacker. After a 45 h incubation, community DNA was extracted using a Qiagen DNeasy PowerSoil extraction kit (cat. no. 12955-4). The 16S rRNA libraries were prepared by PCR amplification with a 515F/806R primer set (16SrRNA_515F: GTG CCA GCM GCC GCG GTA A and 16SrRNA_806R: GGA CTA CHV GGG TWT CTA AT)52 and amplicons sequenced using an Illumina platform to a target depth of 25,000 reads per sample. Sequencing data were analyzed by UNOISE clustering53 (USEARCH v10) and denoising of raw sequences followed by genus-level taxonomic assignments (DADA2/RDP54 v1.6).
Gnotobiotic mouse experiments
All gnotobiotic mouse experiments were performed following Institutional Animal Care and Use Committee and Institutional Biosafety Committee protocols that were approved by the Washington University Animal Studies and Environmental Health and Safety Committees.
Preparation of the 92-strain bacterial consortium
All organisms were cultured in an anaerobic growth chamber (Coy Laboratory Products) under an atmosphere of 77% N2, 20% CO2 and 3% H2. The identity of bacterial stocks was confirmed by sequencing of full-length amplicons generated by PCR of their 16S rRNA genes55 (primers: 8F (AGA GTT TGA TCC TGG CTC AG) and 1391R (GAC GGG CGG TGT GTR CA)). The source and catalog number for each bacterial strain is available in Supplementary Table 4. Organisms were clonally arrayed in a 96-well plate (Sigma-Aldrich, Z707902) and stored at −80 °C in a supplemented Tryptone-Yeast extract-Glucose (TYGs) medium (Supplementary Table 10) containing 15% (vol:vol) glycerol56. The plate was moved into the anaerobic chamber, and a 20 µl aliquot from each well was transferred to 600 µl of TYGs medium in a 96-well deep-well plate (Thermo Fisher, 260251). The deep-well plate was sealed with an aluminum foil cap and incubated anaerobically at 37 °C for 24 h. Growth in each well was assessed by measuring the optical density of aliquots at 600 nm. A 120 µl aliquot from each well was collected, and aliquots were pooled before transfer into 1.8 ml crimp-top sealing glass vials (Wheaton). The sealed gavage mixture was immediately introduced into gnotobiotic isolators after surface sterilization with chlorine dioxide in the transfer sleeve.
Colonization
Germ-free male C57BL/6J mice (24 mice total; The Jackson Laboratory (000664); 20 weeks of age) were maintained within flexible plastic gnotobiotic isolators (Class Biologically Clean) at 22 °C under a strict 12 h light cycle (lights on at 6:00) and fed an autoclavable mouse chow (Envigo, 2018S) ad libitum. Autoclaved bedding (aspen wood chips, Northeastern Products) was changed weekly. Two days before colonization, mice were switched to the HiSF-LoFV diet19. This diet was produced using cooked human foods as described in a previous publication19, freeze-dried, milled (D90 particle size 980 μm) and pelleted. The diet was sterilized by gamma irradiation (20–50 kGy, Steris). Sterility was confirmed by culturing the diet in TYG medium under aerobic and anaerobic conditions.
Mice (n = 4 animals per cage; total of 8 mice per treatment group) were given the HiSF-LoFV diet ad libitum. SG10 (preparation 2) was dissolved in drinking water to a final concentration of 5% (wt:vol). Supplemented water was sterilized by filtration (0.22 μm diameter polyether sulfone filters; Millipore) and introduced to gnotobiotic isolators after surface sterilization with chlorine dioxide in the transfer sleeve. Mice consumed an average of 5 ml drinking water per day, yielding an average daily dose of 250 mg. Fecal samples were collected directly into sterile 2 ml O-ring sealing screw-top plastic vials (Axygen) and frozen immediately in liquid nitrogen. The percent water in cecal samples collected from colonized mice was determined by the difference in their mass before and after lyophilization (74.7 ± 6.2% in SG10 treated versus 82.0 ± 1.8% in untreated animals (mean ± s.d.; P = 0.0064; two-sided unpaired Welch’s t-test)). A third group of mice were maintained in a germ-free state and treated with the HiSF-LoFV diet and water supplemented with SG10 throughout the experiment.
Gavage and recovery of SG10-coated MFABs from mice
SG10-MFABs were introduced to mice on the morning that they were to be euthanized (9 days after gavage of the bacterial consortium). SG10-MFABs were sterilized in 70% ethanol (vol:vol) twice on a magnetic tube stand before resuspension in HNTB (20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES, pH 7.2), 50 mM NaCl, 0.05% (vol:vol) Tween-20 and 0.01% (wt:wt) bovine serum albumin (BSA; Sigma-Aldrich, A7906)). A pool of 25 × 106 beads in 350 μl of HNTB was prepared for each mouse and the mixture was aliquoted into 1.8 ml crimp-top sealing glass vials. The sealed MFAB solution was immediately introduced into gnotobiotic isolators after sterilization of the vial surface with chlorine dioxide in the transfer sleeve. MFABs were introduced to mice via oral gavage of a 350 μl aliquot.
Beads were recovered at the time of euthanasia 4 h after gavage. Cecal contents were gently squeezed into a 50 ml conical tube and then resuspended in 10 ml of HNTB by pipetting and subsequently by vortexing. The resulting slurry was passed through a 100 μm Nylon filter (Corning, 352360). Beads were isolated from the cecal suspension by centrifugation (500g, 5 min) through Percoll Plus (Cytiva, 17544502) in a 50 ml conical tube. Pelleted beads from each animal were distributed into separate 5 ml sterile Eppendorf tubes and washed at least three times with HNTB on a custom magnetic tube rack until macroscopic particulate debris from intestinal contents were no longer observed. Purified beads were filtered through a cell strainer flow cytometry snap cap (Corning, 352235) and stored in HNTB containing 0.01% (wt:wt) sodium azide at 4 °C until use. Input beads were removed from gnotobiotic isolators after all animals had been gavaged, and stored at 37 °C for 4 h with rotation before their isolation (as above).
Glycosyl linkage analysis was performed directly after recovery of beads from the cell strainer. Beads for neutral monosaccharide composition analysis were purified by fluorescence-activated sorting (FACSAriaIII, BD Biosciences). Aliquots of input beads were sorted throughout the procedure to quantify and monitor sort yield and purity. Sorted beads were centrifuged (1,500g, 5 min), the supernatant was removed and the pelleted beads were transferred to wells of a 0.2 ml 96-well skirted PCR plate (Multimax, 2668). Beads were washed with HNTB using a magnetic plate rack, counted and stored at 4 °C in HNTB plus 0.01% (wt:wt) sodium azide until analysis. Bead aliquots (15 × 103) were subjected to neutral monosaccharide composition analysis by GC–MS (see ‘Neutral monosaccharide composition analysis of MFABs by GC–MS’). The order of analysis was randomized with respect to mouse treatment group. Each sample was subjected to two hydrolyses, derivatizations and analyses.
Microbial community analysis
Determination of absolute abundances of community members
Mouse fecal and cecal samples were snap-frozen in liquid nitrogen and stored at −80 °C until use. Microbial community DNA was extracted as described previously43. Sequencing libraries were generated from purified DNA by tagmentation using the Nextera DNA Library Prep Kit (Illumina, 20018705) and custom barcoded primers57. Balanced libraries were sequenced on an Illumina NextSeq instrument (unidirectional 75 nt reads; 3.18 × 106 ± 2.11 × 105 (mean ± s.d.) reads per sample). Reads were demultiplexed and mapped to (1) genomes from the 92-member input bacterial community, (2) two ‘spike-in’ bacterial genomes for absolute abundance calculation (see directly below) and (3) three ‘distractor’ genomes (Bacteroides fragilis NCTC 9343, National Center for Biotechnology Information (NCBI) accession NC_003228.3; Clostridium perfringens ATCC 13124, NCBI accession NC_008261.1; E. coli D9, NCBI accession ACDL01000000). These steps were performed using custom Perl scripts adapted to use Bowtie 2 in a procedure termed community profiling by sequencing (COPRO-Seq)18,20.
To calculate each bacterial strain’s absolute abundance, an aliquot of two bacterial strains not found in mammalian gut communities or the diet was added to each fecal or cecal sample before DNA extraction21,22 (30 µl of a 2.22 × 108 cells ml−1 suspension of Alicyclobacillus acidiphilus DSM 14558 (Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (DSMZ), 14558) (GenBank assembly accession GCA_001544355.1) and 30 µl of a 9.93 × 108 cells ml−1 suspension of Agrobacterium radiobacter DSM 30147 (DSMZ, 30147) (GenBank assembly accession GCA_000421945.1)). COPRO-Seq outputs a counts table that is normalized to the informative genome size of each bacterial genome; this is used to generate a normalized relative abundance table.
The calculated percent relative abundances of the spike-in genomes were 0.52 ± 0.41% and 0.37 ± 0.29% (mean ± s.d.), respectively. For a given taxon i in sample j, the absolute abundance, expressed as genome equivalents per gram of feces/cecum, was calculated using the normalized relative abundance and the A. acidiphilus spike-in (A. a)
$${{{\mathrm{taxa}}}}_{i,\,j}=\frac{{{{\mathrm{relative}}\; {\mathrm{abundance}}}}_{i,\,j}}{{{{\mathrm{relative}}\; {\mathrm{abundance}}\,{\rm{A.}{\,a}}}}_{j}}\times \frac{{{\rm{A.}{\,a}}\;{{\rm{cells}}\; {\mathrm{added}}\; {\mathrm{to}}\; {\mathrm{sample}}}}_{j}}{{{{\mathrm{sample}}\; {\mathrm{mass}}}\,({\mathrm{g}})}_{j}}.$$
Fifty-six community members were identified as having a mean percent relative abundance greater than 0.05% at any one time point in either group and were defined as having colonized the mice. The most abundant distractor genome at any time point in either group was E. coli D9 (0.0003 ± 0.0001 (mean ± s.d.) percent relative abundance). Total community abundance was calculated as the sum of all 56 members. Bacteria with statistically significant SG10 treatment-associated changes in their absolute abundance in the cecal microbiota were identified using one-way ANOVA with the R (v4.1.0) package Stats (v4.1.0). Bacteria with SG10 treatment-associated changes in their fecal levels were identified using a linear mixed-effects model (Gaussian) of absolute abundance data (R package lme4 (v1.1-27.1); using ‘abs abundance ~ group*day + [1|mouse]’). The dependence of bacterial abundance on ‘diet by day’ was tested, and ‘mouse’ was included as a random variable. Tukey’s HSD P values from the linear models were corrected for multiple hypotheses by the Benjamini–Hochberg method. Estimated marginal means of the model were calculated using the R package emmeans (v1.7.2).
Annotation of CAZymes and mcSEED metabolic pathways represented in the genomes of bacterial colonizers of gnotobiotic mice
CAZymes were annotated following the CAZy classification scheme31. PULs in Bacteroides were annotated on the basis of the presence of adjacent susC/susD-like genes and retrieved from the PULDB database (September 2022)24. To predict PUL substrates, individual CAZymes were subjected to NCBI BLASTp against UniProtKB/Swiss-Prot, a curated resource of experimentally characterized proteins58 and PaperBLAST, a regularly updated database of proteins described in published scientific articles59. The enzymatic activity of individual CAZymes were predicted on the basis of the experimentally defined activity of the nearest neighbor (percent amino acid identity). PUL substrates were predicted on the basis of the cumulative activities within that PUL and known glycan structures. Where possible, predicted PUL substrates were confirmed with published microbial gene expression data collected in the presence of a defined glycan carbon source.
In silico predictions of the metabolic pathways of bacterial consortium members were based on an approach, implemented in the RAST/SEED platform, that combines homology- and genome context-based evidence with known sets of enzymatic reactions and nutrient transporters into ‘microbial community (mc) subsystems’; these mcSEED subsystems capture and project variations in particular metabolic pathways across thousands of microbial genomes32. Using this comparative genomics approach, we identified intracellular catabolic pathways and uptake transporters for various carbohydrates, amino acids, other energy sources and vitamins. The results of in silico reconstructions of carbohydrate catabolic pathways are summarized in Supplementary Table 6 in the form of a binary phenotype matrix (BPM). The BPM consists of 55 columns each representing the capacity to use a carbohydrate nutrient, and rows comprising bacterial strains. Each cell in the BPM gives a prediction, based on genomic sequence, of whether that organism possesses the capacity to perform the indicated metabolic function. As such, the BPM also provides a digital phenotype for an organism, where ‘1’ or ‘0’ represents the ability or inability, respectively, to utilize the carbohydrate.
Microbial RNA-seq
Cecal contents were collected at the time of euthanasia, snap-frozen in liquid nitrogen and stored at −80 °C. Monocultures of B. intestinalis DSM 17393 in a defined Bacteroides culture medium46 supplemented with d-glucose, SBABN or SG10 (final concentration 5 mg ml−1) were grown at 37 °C under anaerobic conditions in 12 ml round-bottom culture tubes. Growth was monitored on the basis of optical density measurements at 600 nm using a plate reader, with aliquots of the cultures placed in 96-well flat-bottom plates. At mid-log, cells were collected by centrifugation (7,000g, 7 min; 250 μl for d-glucose and SBABN cultures, 500 μl for SG10 culture). Cells were washed with 500 μl RNAprotect Bacteria Reagent (Qiagen, cat. no. 76506) plus 1% (vol:vol) β-mercaptoethanol, incubated at 22 °C for 5 min and pelleted by centrifugation (7,000g, 7 min), and decanted cells were stored at −80 °C until RNA extraction.
Cecal aliquots (8–25 mg) or collected cell pellets were thawed on ice, and RNA was isolated by first bead beading each sample for 4 min with 250 µl of a slurry of 0.1-mm-diameter zirconia/silica beads, one 3.97 mm steel ball, 500 µl of phenol:chloroform:isoamyl alcohol (25:24:1, pH 7.8–8.2), 210 µl of 20% (wt:wt) SDS and 500 µl of 2× Qiagen buffer A (200 mM Trizma base, 200 mM NaCl and 20 mM EDTA). Samples were then centrifuged (3,220g, 4 min, 4 °C), and 200 µl of the resultant aqueous phase (crude nucleic acid) was transferred to a 96-well deep-well plate along with 140 µl of isopropanol and 20 µl of 3 M sodium acetate (pH 5.5). The solution was mixed by pipetting ten times. Crude nucleic acid was precipitated by incubation at −20 °C for 1 h and recovered by centrifugation (3,220g, 15 min, 4 °C). Pelleted crude nucleic acid was resuspended in 300 µl of Qiagen Buffer RLT by pipetting up and down 50 times. The entire resuspended pellet was transferred from each well to a Qiagen AllPrep 96 DNA plate (Qiagen, 80311). The plate was centrifuged (3,220g, 1 min, 22 °C), and the RNA-containing flow-through was captured and subsequently purified using a Qiagen AllPrep 96 DNA/RNA Kit according to the manufacturer’s protocol. Purified RNA was quantified (NanoDrop) and diluted to 5 ng µl−1. RNA quality was assessed using an Agilent 4200 TapeStation with Agilent High Sensitivity RNA ScreenTape (Agilent, 5067-5579).
RNA-seq libraries were prepared using the Illumina Stranded Total RNA Prep, Ligation with Ribo-Zero Plus Kit (Illumina; 20040529) according to the manufacturer’s protocol that was miniaturized, adapted and optimized for liquid handling robotics. For mouse cecal samples, 55 ng of input RNA was processed, while for B. intestinalis monocultures 1.2 ng of input RNA was processed. Briefly, rRNA was depleted with Ribo-Zero Plus and the rRNA-depleted RNA was isolated using RNACleanXP magnetic beads. The resulting RNA was fragmented and denatured, and the first strand of cDNA was synthesized, followed by synthesis of the second strand; the resulting double-stranded cDNA was purified with AMPure XP magnetic beads, 3′ adenylated, ligated with RNA index anchors, purified with AMPure XP magnetic beads and then amplified with IDT for Illumina Nextera DNA UD Indexes, Set D (Illumina, 20025082), for 16 cycles. The amplified PCR product was purified with AMPure XP magnetic beads, quantified using the Invitrogen Qubit dsDNA BR Assay Kit (ThermoFisher Scientific, Q32853), and libraries of equal mass generated before shallow sequencing on an Illumina MiniSeq-Mid-300 run. The library was rebalanced on the basis of uniquely mapping reads per nanogram of input to target an equal number of uniquely mapping reads per sample, and balance was assessed with an Illumina MiniSeq-Mid-300 run. Balanced libraries were sequenced on an Illumina NovaSeq 6000 with an S4 flow cell (bidirectional 150 nt reads; 74.43 × 106 ± 17.65 × 106 (mean ± s.d.) total reads per cecal sample, 19.26 × 106 ± 1.47 × 106 total reads per B. intestinalis monoculture sample).
Raw sequencing reads were demultiplexed, adapter sequences were removed (Trim Galore v0.6.4; Cutadapt60 v1.16), and STAR61 (v2.7.2b) was used to map uniquely aligning reads to the annotated genomes from the bacterial consortium gavaged into mice (20.49 × 106 ± 1.97 × 106 (mean ± s.d.) uniquely mapped protein-coding reads per cecal sample, 4.23 × 106 ± 4.92 × 105 uniquely mapped protein-coding reads per B. intestinalis monoculture sample). Pre- and post-trimmed sequence quality and potential adapter contamination were assessed using FASTQC62 (v0.11.7). Differential expression analysis was performed using the R package DESeq233 (v1.32.0) on a per-organism basis to identify gene expression levels that were significantly different between treatment groups. Only genomes with more than 10% of their known/predicted genes having greater than 10 raw read counts in at least 4 mice in both experimental groups were analyzed by DESeq2 (33 bacteria met these criterion). Known or predicted genes were annotated using available GFF files (Supplementary Table 4), and our reference collection of metabolic pathways reconstructed in the SEED platform. CAZymes and PULs were annotated by employing custom scripts with manual curation.
Differentially expressed PULs were identified in each organism using GSEA from the R package fgsea34 and the ‘FGSEA-simple’ method (v1.18.0). DESeq2 log2 fold-change values were rank ordered and used as the input. Only PULs with more than five quantified genes were analyzed. Loci plots were generated with the R package genoplotR63 (v0.8.11), and gene orthology was determined with reciprocal NCBI BLASTp.
GC–MS of SCFAs
Frozen cecal contents were weighed in 2 ml screw-top glass vials to which 10 μl of a mixture of internal standards was added (20 mM of acetic acid-13C2,2H4, propionic acid-2H4, butyric acid-13C4, lactic acid-2H3 and succinic acid-13C4). The sample was acidified with 20 μl of 33% (wt:wt) hydrochloric acid. Diethyl ether (1 ml) was added, and the sample was vortexed vigorously for 10 min and then centrifuged (4,000g, 5 min, 22 °C) to separate the mixture into two phases. The upper organic layer was removed and transferred to a clean glass vial before the sample was reextracted with 1 ml of diethyl ether. After combining the two ether extracts, a 60 μl aliquot was mixed with 20 μl of N–tert-butyldimethylsilyl-N-methyltrifluoroacetamide derivatization reagent and allowed to react for 2 h at 22 °C in a GC autosampler vial with a 100 μl glass insert. Derivatized samples were analyzed by a split (1:100 ratio) 1 μl injection on an Agilent 7890A GC system equipped with an Agilent HP-5MS UI capillary column (30 m × 0.25 mm inner diameter (i.d.) × 0.25 µm film thickness; 19091S-433UI) coupled to an Agilent 5977B mass spectrometer detector using electron impact ionization (70 eV) and scanning mode. Helium was used as a carrier gas at a constant flow rate of 1 ml min−1, and the solvent delay time was 3.5 min.
MFAB synthesis, in vitro use and analysis
Synthesis of oligosaccharide-coated MFABs
Paramagnetic, 10-μm-diameter glass beads (Millipore Sigma PureProteome NHS Flexibind, LSKMAGN01) were incubated at 22 °C overnight in a solution of 20 mM HEPES (pH 7.2) and 100 mM NaCl. Surface amine and phosphonate functional groups were installed by incubation with equal molar amounts of (3-aminopropyl)triethoxysilane (Sigma-Aldrich, 440140) and 3-(trihydroxysilyl)propyl methylphosphonate (Sigma-Aldrich, 435716) in deionized water for 5 h at 50 °C with shaking. Beads were derivatized at a density of 5 × 106 beads ml−1, and the organosilane reagents were included at 1,000-fold excess of what would be required to coat the bead surface64. The bead surface was labeled with 100 nM Alexa Fluor 488 NHS ester (ThermoFisher Scientific, A20000) (3 × 106 beads ml−1) in 20 mM HEPES (pH 7.2) and 100 mM NaCl for 50 min at 22 °C. Oligosaccharides conjugated to the bead surface (SG10 or maltodextrin (Sigma-Aldrich, 419680)) were resuspended at 50 mg ml−1 in 50 mM HEPES (pH 7.8) with heat and sonication. Oligosaccharides were activated to a cyanate ester by addition of CDAP (Sigma-Aldrich, RES1458C) (0.1 mg CDAP per milligram oligosaccharide; 350 mg ml−1 dissolved in dimethyl sulfoxide (DMSO); 1 equiv.) in the presence of triethylamine (0.5 equiv.). The oligosaccharide/CDAP/triethylamine solution was mixed for 2 min at 22 °C (refs. 43,44). Alexa Fluor 488-labeled amine plus phosphonate beads that had been resuspended in 50 mM HEPES (pH 7.8) were added to the activated polysaccharide solution, and the reaction was allowed to proceed for 15 h at 22 °C with rotation. Bead density during conjugation was ~20 × 106 beads ml−1, and the final polysaccharide concentration was 43 mg ml−1. Oligosaccharide-conjugated beads were resuspended by bath sonication and reduced by addition of sodium cyanoborohydride (1 equiv.) dissolved in 20 mM HEPES (pH 7.2) and 100 mM NaCl (100 mg ml−1) and incubation for 40 min at 40 °C. Each of the reactions described above was terminated by repeated washing with deionized water on a magnetic tube rack. Washed beads were stored in 20 mM HEPES (pH 7.2) and 100 mM NaCl at 4 °C until use.
Bead counting by flow cytometry
Beads were resuspended with vortexing and bath sonication. Typically, 5 μl of a bead solution were added to 200 μl of HNTB containing 2 μl of CountBright Absolute Counting Beads (ThermoFisher Scientific, C36950). Beads were analyzed using flow cytometry on a FACSAriaIII instrument (BD Biosciences), and the data were analyzed using FlowJo (v10.8.0). Absolute bead density in the stock solution was determined according to the manufacturer’s suggested protocol. An example of the gating strategy is shown in Supplementary Fig. 3.
In vitro enzymatic digestion of oligosaccharide-coated MFABs
Porcine α-amylase (Megazyme, E-PANAA), Aspergillus niger amyloglucosidase (Megazyme, E-AMGFR), Aspergillus niger endo-1,5-α-arabinanase (Megazyme, E-EARAB), Cellvibrio japonicus α-L-arabinofuranosidase (Megazyme, E-ABFCJ) or Neocallimastix patriciarum endo-1,4-β-xylanase (Megazyme, E-XYLNP) were added to oligosaccharide-coated MFABs (2.5 × 106 beads ml−1 for the maltodextrin-MFAB digestion or 5 × 106 beads ml−1 for the SG10-MFAB digestion). Free SG10 was digested at a concentration that equaled the concentration of MFAB-bound SG10 (0.15 mg ml−1). All reactions contained a total of 1.2 units of enzyme (per the manufacturer’s documentation) per 1 × 106 beads. For digestion of SG10 (MFAB-bound or free), reactions were performed in 100 mM sodium acetate (pH 4.0) and 0.5 mg ml−1 BSA (wt:wt) at 37 °C for 24 h with rotation to allow maximum possible degradation. For digestion of maltodextrin-MFABs, all reactions were performed in 50 mM sodium malate (pH 6) and 2 mM calcium chloride at 37 °C for 4 h with rotation (in the case of the arabinanase digestion, we used 100 mM sodium acetate/BSA per the manufacturer’s recommendation). Reactions were terminated with repeated washing in 20 mM HEPES (pH 7.2) and 100 mM NaCl on a magnetic tube rack before heat inactivation at 90 °C for 15 min. Beads were then washed repeatedly in 20 mM HEPES (pH 7.2) and 100 mM NaCl on a magnetic tube rack and stored at 4 °C until analysis. Digestions of free SG10 were heat inactivated as described above and centrifuged (15,000g, 15 min) to clear precipitated protein. The resulting supernatant was removed and stored at −20 °C before performing the MS analyses described ‘Neutral monosaccharide composition analysis of MFABs by GC–MS’.
Neutral monosaccharide composition analysis of MFABs by GC–MS
An aliquot of beads (15 × 103–25 × 103) were washed repeatedly with deionized water in a 96-well skirted PCR plate (Multimax) using a magnetic plate rack (Axygen, IMAG96P). Beads were resuspended in 175 μl of 2 M trifluoroacetic acid (TFA) containing 15 ng of D6-myo-inositol (CDN Isotopes, D3019) and transferred to 8 mm crimp-top sealing glass vials (Fisher Scientific, C4008-632C) before capping with Teflon-coated aluminum caps (Fisher Scientific, C4008-2A). A 5 μl aliquot of the bead solution was removed before capping and used to count the number of beads that would be subjected to hydrolysis.
Oligosaccharides were hydrolyzed to monosaccharides by incubation at 95 °C for 2 h. Glass vials were centrifuged (3,200g, 5 min), the supernatant was transferred to a new glass vial, and the sample was dried with reduced pressure using a centrifugal vacuum concentrator. Samples were oximated with addition of 20 μl of methoxyamine (15 mg ml−1 in pyridine) and incubation at 37 °C overnight. Twenty microliters of N-methyl-N-trimethylsilyl-trifluoroacetamide plus 1% chlorotrimethylsilane (ThermoFisher Scientific, TS-48915) were added, and the solution was incubated at 70 °C for 1 h. The sample was diluted with 20 μl of heptane before analysis using an Agilent 7890A GC system equipped with an Agilent HP-5MS UI capillary column (30 m × 0.25 mm i.d. × 0.25 µm film thickness; 19091S-433UI) coupled to an Agilent 5975C mass spectrometer detector with a splitless 1 μl injection and electron ionization (EI) in scan mode. A dilution series of l-arabinose, d-galactose, d-glucose, d-mannose, d-rhamnose and d-xylose standards was used to identify corresponding peaks and to generate standard curves for quantitation. Raw. AIA files were exported from the GC–MS instrument, and peaks were identified and quantitated using the R package metaMS65 (v1.28.0). Peak areas were corrected using a D6-myo-inositol internal standard, and absolute quantitation was determined from linear fits of 2-fold diluted standards. The mass of oligosaccharide bound to MFABs was derived from the quotient of the absolute mass of monosaccharide and the number of beads subjected to acid hydrolysis. The percent mass remaining was calculated as the quotient of the absolute mass of each sample and the mean of the reference sample. Graphs were generated and statistical analysis was performed using R (v4.1.0). Independent sample preparations represent independent GC–MS derivatizations and analyses from the same sample.
Carbohydrate structural and quantitative analyses
Monosaccharide composition analysis of conditioned culture medium by GC–MS
A 20 µl aliquot of conditioned culture medium (corresponding to 100 µg of carbohydrate at the t = 0 time point) was added to 20 µg inositol internal standard (1 mg ml−1 in deionized water) and dried using a centrifugal vacuum concentrator. Samples were hydrolyzed in 2 M TFA for 2 h at 120 °C, reduced with sodium borodeuteride (10 mg ml−1 dissolved in 0.1 M ammonia) overnight and acetylated using acetic anhydride/TFA. The derivatized material was extracted with dichloromethane, washed with deionized water and concentrated to a volume of ~300 µl in dichloromethane using a nitrogen gas stream.
Samples were analyzed on an Agilent 7890A gas chromatograph equipped with a 5975C mass spectrometer detector (EI mode with 70 eV). A 1 µl sample was injected in split mode (1:10 ratio), and peaks were detected by scanning an m/z range between 30 and 450 with a scan rate of 3.4 s−1 and gain factor of 1. Independent sample preparations represent independent GC–MS derivatizations and analyses from the same sample. Peak areas were normalized to the inositol internal standard signal from that injection, and the amount of monosaccharide remaining at each time point was calculated relative to the t = 0 time point.
Solution-based glycosyl linkage analysis of free oligosaccharides
Glycosyl linkage analysis of oligosaccharides was performed as described previously with minor modifications66. Briefly, a glycan solution of 20–100 µg was lyophilized or dried using a centrifugal vacuum concentrator, dissolved in DMSO for 30 min with gentle agitation, then freshly prepared sodium hydroxide slurry was added and incubated for 10 min followed by addition of iodomethane and incubation for 40 min. The permethylated sample was subsequently extracted, washed and blown dry with nitrogen gas. The sample was hydrolyzed using 2 M TFA plus 1 µg inositol internal standard for 2 h at 120 °C, reduced with sodium borodeuteride (10 mg ml−1 dissolved in 0.1 M ammonia) overnight and acetylated using acetic anhydride/TFA. The derivatized material was extracted with dichloromethane, washed with deionized water and then concentrated to a volume of ~100 µl in dichloromethane using a nitrogen gas stream. For glycosyl linkage analysis of conditioned culture medium, 40 µl of the medium was dried (corresponding to 200 µg of carbohydrate at the t = 0 time point of the incubation) and analyzed without modification.
Samples were analyzed on an Agilent 7890A gas chromatograph equipped with a 5975C mass spectrometer detector (EI mode with 70 eV), using a 30 m RESTEK RTX-2330 capillary column (30 m × 0.25 mm i.d. × 0.25 µm film thickness). The temperature of the injector was 250 °C. The temperatures of the detector were 230 °C for the source and 150 °C for the quadrupoles. The GC temperature program used was 80 °C for 2 min, followed by a ramp of 30 °C min−1 to 170 °C, then a second ramp of 4 °C min−1 to 245 °C and a final holding time of 5 min. The helium flow rate was 1 ml min−1, and the sample injection was 5 µl in pulsed splitless mode with 50 psi for 2 min. Linkage peak detection of samples was carried out by either selective ion monitoring mode (see ‘MFAB-based glycosyl linkage analysis’ section) or scanning an m/z range between 30 and 450 with a scan rate of 3.4 s−1 and a gain factor of 3. Peak areas were normalized to the inositol internal standard signal from that injection. Independent sample preparations represent independent GC–MS derivatizations and analyses from the same sample.
MFAB-based glycosyl linkage analysis
SG10-MFABs were washed thoroughly with 2 ml of deionized water five times on a magnetic tube rack. The sample was uniformly resuspended in 0.2–1.5 ml of deionized water and aliquoted into 2 ml screw-top vials containing 20–60 μg of oligosaccharide (determined by neutral monosaccharide composition analysis). Samples were lyophilized to dryness on a magnet and stored under vacuum at 22 °C until analysis.
For derivatization, an SG10-MFAB aliquot was resuspended in 600 µl of DMSO and divided equally into three glass vials to analyze in triplicate. The MFAB–DMSO suspensions were gently agitated for 30 min with sonication for 1 min after 15 and 30 min. Two-hundred microliters of a freshly prepared sodium hydroxide slurry66 was added to each suspension and gently agitated for 10 min, followed by addition of 100 µl of iodomethane before incubation for 40 min with gentle agitation. Mixtures were sonicated for 1 min every 10 min. Beads were separated using a magnetic tube rack, and the supernatant was removed. Beads were then washed once with 300 µl DMSO, followed by 300 µl of deionized water three times. Beads were transferred to a new glass vial, the liquid was removed and the wet beads were dried with a stream of nitrogen gas. The permethylated MFAB samples were then processed as described above, except that a phase separator was used to collect the organic layer and to remove the beads.
The derivatized samples were analyzed on an Agilent 7890A gas chromatograph equipped with a 5975C mass spectrometer detector as described above with slight modifications. The sample injection was 3 µl in pulsed splitless mode with 50 psi for 2 min. Linkage peak detection of SG10 was performed by selective ion monitoring with a gain factor of 3 based on the signature fragments of each linkage (Supplementary Table 8). Data collection began at 9 min; at each time point, a new set of ion monitoring was enabled. Peak areas were normalized to the inositol internal standard signal from that injection. Independent sample preparations represent independent GC–MS derivatizations and analyses from the same sample.
2D (1H,13C)-HSQC NMR
A 2 mg aliquot of lyophilized SG10 was dissolved in 250 μl of deuterium oxide with 0.1% (vol:vol) acetone as the internal standard (1H-2.22 ppm, 13C-30.89 ppm) plus 0.1% (vol/vol) acetonitrile. The solution was placed into a 3 mm NMR tube, and HSQC spectra were recorded at 25 °C on a Bruker AVANCE III 600 MHz spectrometer equipped with a 5 mm cross-polarization quadruple resonance cryoprobe with Z-axis gradient using the Bruker pulse program HSQCEDETGPSISP2.3. HSQC experiments were performed with eight scans and a 1.5 s recycle delay. Each spectrum was acquired from 7.0 to 0.0 ppm in F2 (1H) with 1024 complex data points and 120 to 0 ppm in F1 (13C) with 256 complex data points. The resulting spectra were analyzed using Bruker TopSpin (v4.1.4) software.
MALDI-TOF MS of oligosaccharides
Following monosaccharide composition analysis, a 15–30 µg aliquot of MFAB-bound oligosaccharide was added to 2 ml O-ring sealing plastic screw-top vials (Axygen), washed extensively with deionized water on a magnetic tube rack, frozen in liquid nitrogen and dried by lyophilization. A total of 300 µl of 1 M NH4OH in deionized water was added to the dried MFAB sample, and the mixture was incubated at 70 °C for 3.5 h with vortexing every 30 min. Released oligosaccharide was separated from beads on a magnetic tube rack and removed. Beads were washed twice with 150 µl of 1 M NH4OH and bath sonicated (Branson). The solution was removed using a magnetic tube rack and combined with the initial collection of released oligosaccharides. Samples were dried under reduced pressure using a centrifugal vacuum concentrator. The sample was resuspended in deionized water and dried again. Free oligosaccharide samples were treated identically as described above. The released oligosaccharide was resuspended in deionized water to a concentration of 0.15 µg µl−1 using heat and bath sonication. The mixture was cleared by centrifugation (12,000g, 10 min) and purified with cation exchange resin (Dowex Monosphere 88 H+) that had been washed with deionized water. The mixture was incubated at 22 °C for 5 min with rotation. Supernatant was transferred to a new vial by pipette and cleared with centrifugation (12,000g, 5 min), and 10 µg of deionized oligosaccharide was aliquoted into glass sample vials with a fixed insert (Agilent Technologies, 5188-6591). Samples were dried with a centrifugal vacuum concentrator for 2 h and stored at 22 °C until analysis. To quantify oligosaccharide release, recovered beads were subjected to neutral monosaccharide composition analysis and compared with input beads never exposed to NH4OH.
Dried samples were resuspended in 4 µl of 10 mg ml−1 Super DHB (Sigma-Aldrich, 50862), dissolved in a 1:1 (vol:vol) mixture of acetonitrile:water. Samples were spotted onto a polished steel 384-spot MALDI plate. MALDI-TOF MS spectra were obtained on a Bruker ultrafleXtreme MALDI-TOF/TOF instrument run in positive reflector mode (instrument method RP 0–700) with no ion suppression or cutoff. Laser intensity was adjusted as needed to keep the signal consistent between samples. Oligosaccharides ionized predominantly as monosodiated adducts. Data were collected as the average of five laser shots, exported as text files and analyzed in the R package MALDIquant67 (v1.20).
Using MALDIquant, data were variance stabilized and smoothed, baseline was corrected and peak intensity was normalized. Spectra were aligned to a 0.002 Da tolerance and a signal-to-noise ratio equal to 2. Peaks were selected with a signal-to-noise ratio greater than 5 and binned to 0.002 Da tolerance. Oligosaccharide peaks, each with one additional monosaccharide residue, were selected in R (v4.1.0) from a list of theoretical monoisotopic masses of monosodiated adducts in a fashion that minimized the difference between theoretical mass and observed peak mass. Only peaks with a mass error less than 100 ppm were included in the analysis; mass errors were typically 10–50 ppm. Peak intensities were normalized to the most abundant peak in each sample to represent changes in oligosaccharide length distribution. MFAB-released oligosaccharides with a 3-aminopropyl linker were never observed in the mass spectrum. While we cannot rule out an intramolecular elimination of the linker, our data suggest that oligosaccharide is released by hydrolysis of the isourea bond.
GH activity assays
Generation of recombinant GHs encoded by B. intestinalis PUL8 and PUL43
A culture of B. intestinalis DSM 17393 was grown overnight in a defined Bacteroides culture medium46 supplemented with 5 mg ml−1 d-glucose (Supplementary Table 11). DNA was isolated from the cell pellet, derived from 0.75 ml of the culture, by using hot shot lysis then the Qiagen PCR Purification Kit (Qiagen, 28104) before storage in deionized water at −20 °C. Oligonucleotide primers for amplifying each open reading frame (as annotated in PATRIC (recently renamed Bacterial and Viral Bioinformatic Resource Center) ID 471870.8) were designed using SnapGene (v7.0.1) to (1) remove predicted signal peptide sequences40 and (2) include overlap with the plasmid insertion site (Supplementary Table 9). Each gene was amplified using Q5 Taq polymerase (New England Biolabs, M0492S), purified (Qiagen, 28104) and inserted into a pET28b vector with an N-terminal His10-tag by HiFi assembly according to the manufacturer’s suggested protocol (New England Biolabs, E2621S). The assembly mixture was transformed into chemically competent E. coli DH5α cells, and plasmid DNA was isolated from single colonies using a miniprep DNA kit (Qiagen, 27104). Plasmid identity was confirmed by whole plasmid sequencing (Plasmidsaurus).
GHs were expressed as N-terminally His10-tagged fusion proteins in E. coli BL21(DE3). To do so, cells were grown in Terrific Broth (Research Products International, T15000) supplemented with 50 μg ml−1 kanamycin to mid-log phase (0.4–0.6 OD600). Protein expression was then induced by addition of isopropyl-β-d-1-thiogalactopyranoside to 0.1 mM or 0.3 mM followed by an 18 h incubation at 20 °C with shaking (expression conditions for each protein are provided in Supplementary Table 9). Cells were isolated by centrifugation (5,000g, 10 min), and the resultant cell pellets were stored at −80 °C until use.
Frozen cell pellets were thawed on ice before resuspension in 5× (wt:vol) 50 mM HEPES (pH 7.4), 5% (wt:wt) glycerol, 250 mM NaCl, 20 mM imidazole, 1 mM phenylmethylsulphonyl fluoride plus 3–5 mg lysozyme (ThermoFisher), 1 mg RNase (Sigma) and 60 units DNase (Sigma) per gram of wet cell pellet. Cell suspensions were rotated for 30 min at 4 °C before lysis by tip sonication (Branson), and cell debris was removed by centrifugation (11,000g, 40 min, 4 °C). The resulting supernatant was filtered and subjected to Ni-NTA affinity chromatography (Cytiva, 17524701) on an AKTA Pure fast protein liquid chromatography system (Cytiva). Proteins were eluted using a linear gradient from 20 mM to 500 mM imidazole in 20 mM potassium phosphate (pH 7.4) and 500 mM NaCl. Individual fractions of eluted protein were visualized by SDS–PAGE (Bio-Rad) and Coomassie staining (Bio-Rad, 1610786). Fractions that were >95% pure (Supplementary Fig. 2) were pooled and buffer exchanged at 4 °C for 36–48 h via dialysis (ThermoFisher, 66810) (dialysis buffer details are provided in Supplementary Table 9). Protein concentration was determined by ultraviolet–visible light spectroscopy (Shimadzu 1900i) at 280 nm using an ε value calculated from the protein’s primary sequence. Recombinant proteins were either stored at 4 °C for immediate use, or concentrated using a Millipore centrifugal filter (10,000 Da molecular weight cutoff; UFC801024) and supplemented with glycerol to 10% (vol:vol) before storage at −80 °C. BACINT_00520, 00524, 00525, 00526, 02466, 02785 and 02768 were purified more than once due to low yield and reaction condition optimization with similar purity and biochemical results. The remaining proteins were purified once and used for all experiments. No soluble protein was recovered from BACINT_00515 despite repeated efforts.
In vitro digestion of arabinan or SG10 by B. intestinalis GHs
The recombinant His10-tagged GHs from PUL8 and PUL43, as well as commercially available enzymes (A. niger endo-1,5-α-arabinanase (Megazyme, E-EARAB), C. japonicus α-l-arabinofuranosidase (Megazyme, E-ABFCJ), A. niger, α-l-arabinofuranosidase (Megazyme, E-AFASE), porcine α-amylase (Megazyme, E-PANAA), N. patriciarum endo-1,4-β-xylanase (Megazyme, E-XYLNP)), were individually incubated at 37 °C with 10 mg ml−1 SBABN, 10 mg ml−1 SG10 or deionized water (no glycan control) at a final enzyme concentration of 0.5 µM in an optimized reaction buffer (Supplementary Table 9). Enzyme reactions were performed in biological triplicate, while the no enzyme, SBABN only control or SG10 only control were performed in biological duplicate. Reactions were terminated at time points 0 min, 30 min and 4 h by heating to 95 °C for 5 min before cooling to room temperature and storage at −20 °C.
The enzymatic activity of each protein was determined using a bicinchoninic acid (BCA) assay45 (ThermoFisher Scientific, 23225) and a coupled enzyme assay that specifically detects l-arabinose or d-galactose (Megazyme, K-ARGA). For the BCA assay, 40 μl of reaction product was added to a skirted 96-well PCR plate (Bio-Rad, HSP9601) before 120 μl of 50:1 (reagent A:reagent B) BCA working reagent was added. Plates were sealed (Bio-Rad, MSB1001) and incubated at 80 °C for 20 min in a thermal cycler. After cooling to room temperature, a 120 μl aliquot was transferred to a 96-well half-area plate (Greiner, 675180) and absorbance was measured at 562 nm. The l-arabinose/d-galactose coupled enzyme assay kit (Megazyme, K-ARGA) was used according to the manufacturer’s suggested protocol. Briefly, 20 μl of the reaction was combined with 10 μl β-nicotinamide adenine dinucleotide, 20 μl reaction buffer and 190 μl water in a 96-well plate (Costar, 3370). After 3 min, 2 μl of β-galactose dehydrogenase and galactose mutarotase was added to each well and mixed by pipette, and absorbance at 340 nm was monitored for 1 h at 1 min intervals. For both assays, (1) a two-fold dilution series of l-arabinose in the enzyme’s corresponding reaction buffer was used to generate a standard curve on the sample plate, and (2) each biological replicate was tested in duplicate. The number of micrograms of relative reducing ends (BCA assay) or l-arabinose (coupled enzyme assay) was calculated from the standard curve, and the 30 min and 4 h time point data were made relative to the 0 min time point. Data were normalized to represent a 50 µl reaction. The normalized data were used for a one-sided (greater than) Mann–Whitney U test comparing product abundance for a given substrate at time 30 min or 4 h to the time-matched no enzyme control samples and corrected by the Benjamini–Hochberg method. The mean of normalized technical replicates was calculated and graphed with ggplot2 (v3.4.4). Statistical analysis was performed in R (v4.2.0).
In vitro bacterial growth assays
Bacterial stocks were struck onto agar plates containing brain–heart infusion medium (Becton Dickinson) supplemented with 10% (vol:vol) horse blood. Plates were incubated anaerobically at 37 °C for 1 day. Single colonies were then picked and grown overnight in a defined Bacteroides culture medium46 (Supplementary Table 11) containing 5 mg ml−1 d-glucose at 37 °C. Bacteria were diluted 1:250 (vol:vol) into 2× defined Bacteroides culture medium and aliquoted into wells of a 384-well plate using a liquid handling robot located within a Coy chamber. Carbon sources were resuspended at 10 mg ml−1 in deionized water and sterilized by autoclaving. Tested carbon sources included d-glucose, SBABN (Megazyme, P-ARAB), SG10 preparation 1 and SG10 preparation 2. An equal volume of the stock solution of a given carbon source was added to each well (final concentration, 5 mg ml−1). Plates were sealed with an optically clear membrane (Axygen, UC500), and growth at 37 °C under anaerobic conditions was monitored by measuring optical density at 600 nm every 15 min (Biotek Eon instrument with a BioStack 4). All conditions were tested in triplicate.
Statistical analyses
Details regarding statistical tests used, number and definition of replicates, means and variance are provided in the text, figure legends or tables. We employed Benjamini–Hochberg correction, ANOVA, linear mixed-effects model (Gaussian), Tukey’s HSD (confidence level 0.95) of ANOVA results, negative binomial generalized linear model, two-sided Welch’s t-test and Mann–Whitney U test (one- or two-sided) statistical tests that were performed in R. Exact P values are reported in the figures, figure legends or source data files. The gnotobiotic mouse experiment was performed one time, with eight mice per group.
Materials availability
Most bacterial strains used in the present study were obtained from ATCC or DSMZ. Strains from the corresponding author’s lab can be obtained upon request.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Related
Yaslen Clemente Shows Off Leg Day Gains and Shares Her…
Yaslen Clemente isn't just an influencer—she's a fitness powerhouse. The social media star is known for her intense workouts, and she recently sha
Samantha Espineira Stuns in Blue Swimsuit and Shares Her 5…
Samantha Espineira knows how to turn heads, both on and off the runway. The successful model and Instagram influencer regularly shares breathtaking
The Best Fitness Trackers To Help You Reach Any Health…
Best Health Tracker: Oura Ring 3Why We Love It: I’ve tried many, many fitness trackers—but I tend not to stick with one watch or band for very long. I’ve
#CycleSyncing debunked: Popular TikTok trend not backed by science
A new study has debunked a popular TikTok wellness trend called cycle syncing, which claims that tailoring a workout routine to match the hormonal changes that












